Description
The SARS-CoV-2 Rapid Antibody Test is a reliable, rapid chromatographic immunoassay intended for qualitative detection of antibodies (IgM and IgG) to SARS-CoV-2 in human serum, plasma or whole blood.
The SARS-CoV-2 Rapid Antibody Test is intended for the use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating prior infection. This rapid lateral flow test is intended for professional use in laboratory and near patient-testing environments and qualitatively detects IgM and IgG specific to SARS-CoV-2 in serum, plasma, and whole blood.
SARS-CoV-2: An overview of virus structure, transmission and detection
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is an enveloped, single-stranded RNA virus of the family Coronaviridae. Coronaviruses share structural similarities and are composed of 16 nonstructural proteins and 4 structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N). Coronaviruses cause diseases with symptoms ranging from those of a mild common cold to more severe ones such as Coronavirus Disease 2019 (COVID-19) caused by SARS-CoV-2 1,2.
SARS-CoV-2 is transmitted from person-to-person primarily via respiratory droplets, while indirect transmission through contaminated surfaces is also possible3-6. The virus accesses host cells via the angiotensin-converting enzyme 2 (ACE2), which is most abundant in the lungs7,8.
The incubation period for COVID-19 ranges from 2 – 14 days following exposure, with most cases showing symptoms approximately 4 – 5 days after exposure3,9,10. The spectrum of symptomatic infection ranges from mild (fever, cough, fatigue, loss of smell and taste, shortness of breath) to critical11,12. While most symptomatic cases are not severe, severe illness occurs predominantly in adults with advanced age or underlying medical comorbidities and requires intensive care. Acute respiratory distress syndrome (ARDS) is a major complication in patients with severe disease. Critical cases are characterized by e.g., respiratory failure, shock and/or multiple organ dysfunction, or failure11,13,14.

- Nucleocapsid protein (N)
- Envelope protein (E)
- Spike protein (S)
- Membrane glycoprotein (M)
- RNA
The benefit of having a
SARS-CoV-2 antibody test available
Antibody tests are crucial for patient contact tracing, defining previous exposure, and for epidemiological studies. Epidemiological studies are urgently needed to help uncover the spread and burden of disease, in particular, the rate of asymptomatic infections, and to get better estimates on morbidity and mortality. This test may also be used together with molecular tests, like Roche’s cobas® SARS-CoV-2 PCR test, to aid in the diagnosis of suspected COVID-19 patients. As more is understood about immunity to SARS-CoV-2, it may help to assess who has built up immunity to the virus helping healthcare providers better manage patients.
Features and benefits of the SARS-CoV-2 Rapid Antibody Test
The SARS-CoV-2 Rapid Antibody Test can help relieve the burden on healthcare infrastructure generally by making reliable accurate testing possible in decentralised locations, complementing laboratory diagnostics and enabling effective screening. This allows for widespread mass screening without the need to over-burden specialist staff and laboratories. Patients can get a quick and convenient answer that in combination with local and national policies will help them modify their behaviour and help contain the virus and optimise restriction strategies. Follow-up appointments between patients and doctors to discuss the test results can be avoided.
One of the key benefits of the SARS-CoV-2 Rapid Antibody Test is that it can be performed with a small capillary blood sample (20 μl) from the fingertip. It opens the door to testing even for elderly and infirm individuals for whom a venous blood draw is not viable or convenient. And because this rapid test delivers reliable results, clinicians can trust on the results and advise patients with confidence43.
The benefits of the SARS-CoV-2 Rapid Antibody Test in short:
- Getting a quick result (10-15 minutes) – no need for follow-ups to discuss test results
- Easy handling which does not require specific training
- Only needing a very small capillary sample of 20ul
- Access to testing in areas where laboratory testing is not available, or when a venous blood draw is not suitable.
SARS-CoV-2 Rapid Antibody Test handling

Testing the quick and easy way
Testing process for the SARS-CoV-2 Rapid Antibody Test
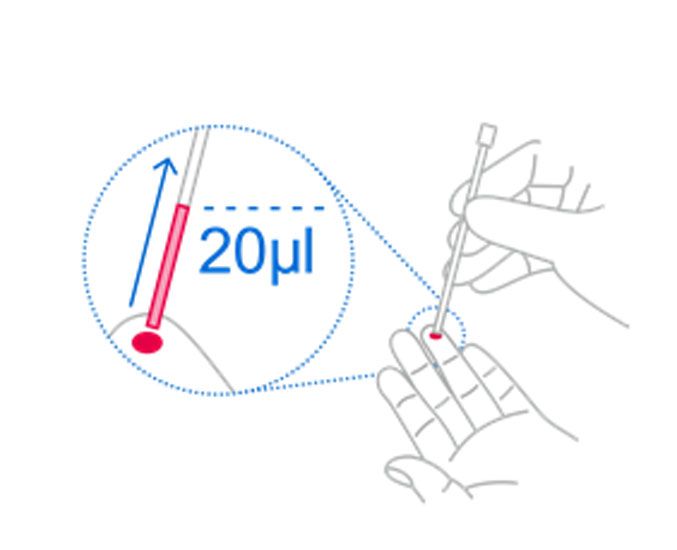
1. Take finger stick blood
Using a capillary tube, collect 20 μl of capillary whole blood to the black line of the capillary tube.
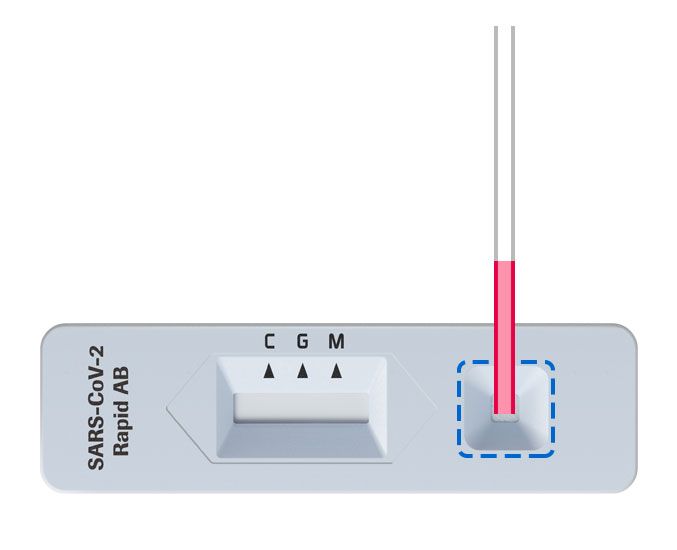
2. Add blood to test
Add the collected capillary whole blood to the specimen well of the test device.
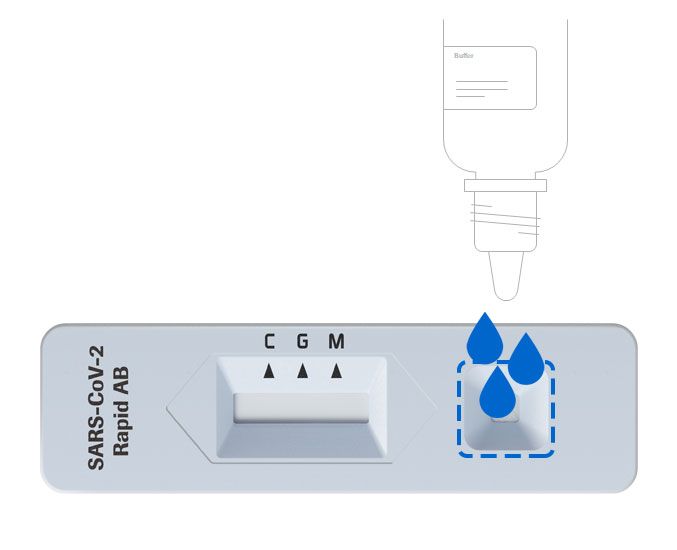
3. Drop of buffer
Add 3 drops (90μl) of buffer vertically into the specimen well of the test device.
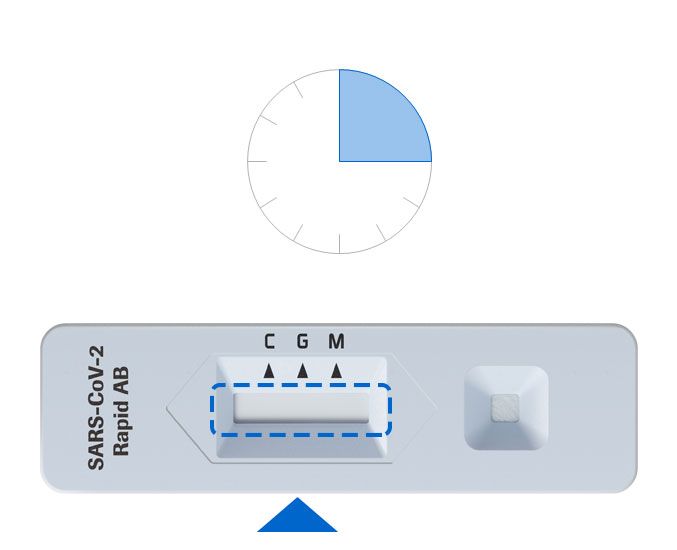
4. Read the result
Read the test result at 10-15 minutes.
Do not read test result after 15 minutes. It may give a false result.
Test kit information
The kit is ready for use and contains all equipment needed to perform a test, with the exception of a pricking device (lancet), which is not included.
A pricking device (lancet) is needed if a blood sample is taken from the finger or ear. Roche recommends to use the “Accu-Chek Safe T Pro Uno or Plus” which can be ordered through the usual channels. Any other pricking device that is able to produce a sample of 20 microliters can be used too.
The following components are needed for a test and included in the kit:
- Test device (individually in a foil pouch with desiccant)
- Buffer bottle
- Capillary tube (20μl) (optional)
- Instructions for use
- Quick Reference Guide

Roche’s response to the COVID-19 pandemic
Our commitment to help put a stop to the COVID-19 pandemic
References
- Su, S. et al. (2016). Trends Microbiol. 24(6), 490-502.
- Zhu, N. et al. (2020). N Engl J Med. 382(8), 727-733.
- Chan, J.F. et al. (2020). Lancet. 395, 514-523.
- U.S. CDC. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html. Published April 2, 2020. Accessed April 15, 2020.
- WHO. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Published March 29, 2020. Accessed April 15, 2020.
- Kampf, G. et al. (2020). J Hosp Infect. 104(3), 246-251.
- Letko, M. et al. (2020). Nat Microbiol. 5, 562-5.
- Hoffmann, M. et al. (2020). Cell. 181, 271-80.e8.
- WHO. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200403- sitrep-74-covid-19-mp.pdf. Published April 3, 2020. Accessed April 15, 2020.
- Lauer, S.A. et al. (2020). Ann Intern Med. 172(9), 577-82.
- Rothe, C. et al. (2020). N Engl J Med. 382(10), 970-971.
- Kupferschmidt, K. Study claiming new coronavirus can be transmitted by people without symptoms was flawed. Science. https://www.sciencemag.org/news/2020/02/paper-non-symptomatic-patient-transmitting-coronavirus-wrong. Published February 4, 2020. Accessed April 15, 2020.
- Bai, Y. et al. (2020). JAMA. 323(14), 1406-1407.
- Mizumoto, K. et al. (2020). Euro Surveill. 25(10), 2000180.
- Hu, Z. et al. (2020). Sci China Life Sci. 63(5), 706-711.
- U.S. CDC. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Published March 20, 2020. Accessed April 15, 2020.
- Wang, D. et al. (2020). JAMA. 323(11), 1061-1069.
- Huang, C. et al. (2020). Lancet. 395(10223), 15-2.
- Arentz, M. et al. (2020). JAMA. 323(16), 1612-1614.
- Wu, Z. et al. JAMA. 323(13), 1239-1242.
- WHO. https://apps.who.int/iris/bitstream/handle/10665/331501/WHO-COVID-19- laboratory-2020.5-eng.pdf. Published March 19, 2020. Accessed April 15, 2020.
- U.S. CDC. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published March 14, 2020. Accessed April 15, 2020.
- EUCDC. https://www.ecdc.europa.eu/sites/default/files/documents/Overview-rapid-test-situation-for-COVID-19-diagnosis-EU-EEA.pdf. Published April 1, 2020. Accessed April 15, 2020.
- WHO. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/. Published April 11, 2020. Accessed April 15, 2020.
- Liu, W. et al. (2020). J Clin Microbiol. 58(6), e00461-2.
- To, K. et al. (2020). Lancet Infect Dis. 20(5), 565-74.
- Long, Q. et al. (2020). medRxiv. https://doi.org/10.1101/2020.03.18.20038018.
- Lou, B. et al. (2020). Eur Resp J. https://doi.org/10.1183/13993003.00763-2020.
- Zhao, J. et al. (2020). Clin Infect Dis. pii: ciaa344. https://doi.org/10.1093/cid/ciaa344. Accessed November 18, 2020
- Zhang, B. et al. (2020). medRxiv. https://doi.org/10.1101/2020.03.12.20035048.
- Wölfel, R. et al. (2020). Nature. 581, 465-469.
- Xiao, D.A.T. et al. (2020). J Infect. 81(1), 147-178.
- Tan, W. et al. (2020). medRxiv. https://doi.org/10.1101/2020.03.24.20042382.
- Okba, N. et al. (2020). medRxiv. https://doi.org/10.1101/2020.03.18.20038059.
- Alberts, B. et al. (2002). Molecular Biology of the Cell. 4th edition. New York: Garland Science. B Cells and Antibodies. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26884/
- Klasse, P.J. (2016). Expert Rev Vaccines 15(3), 295-311.
- Payne, S. (2017). Viruses: Chapter 6 – Immunity and Resistance to Viruses, Editor(s): Susan Payne, Academic Press, Pages 61-71, ISBN 9780128031094.
- Iwasaki, A. and Yang, Y. (2020). Nat Rev Immunol. https://doi.org/10.1038/ s41577-020-0321-6.
- Amanat, F. et al. (2020). Nat Med. https://doi.org/10.1038/s41591-020-0913-5.
- Zhou, P. et al. (2020). Nature. 579(7798), 270-273.
- Haveri, A. et al. (2020). Euro Surveill. 25(11), 2000266.
- Poh, C. et al. (2020). bioRxiv. preprint doi: https://doi.org/10.1101/2020.03.30.015461.
- SARS-CoV-2 Rapid Antibody Test. Package Insert 2020-07, V1.0; Material Number 09216448190





Reviews
There are no reviews yet.